Yes, that is the periodic table displayed above, but it is presented a little bit differently than you have usually seen it:
• Elements 57 through 70 (and 89 through 102) are usually shown separately, and at the bottom of the chart.
Here, they are placed right alongside the other elements in numerical order.
• All the elements are shown with 4 background colors: blue, yellow, green or red.
By being displayed in this way, it is easy to see how the orbitals of each element get "filled in" with electrons.
Let's look at the first 3 rows of the periodic table.
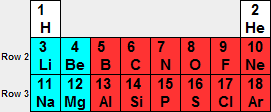
Row 2 consists of the 2s (blue) and 2p (red) orbitals.
Row 3 consists of the 3s (blue) and 3p (red) orbitals.
This pattern continues for all the rows thereafter.
When we move down to Rows 4 and 5, the 3d and 4d orbitals (green) also get filled with electrons.

|
| |
Moving down to Rows 6 and 7, the 4f and 5f orbitals (yellow) get filled with electrons.

Here's the above 3 graphics combined.

Now let's show the periodic table with the names of the orbitals ("s" "p" "d" and "f") replacing the element numbers and symbols:

| |
You probably remember that the "s" orbital contains 2 electrons, the "p" orbital 6, the "d" orbital 10 and the "f" orbital 14.
Now, let's see how all of this comes together.
Row 1 contains 2 electrons in the 1s orbital, row 2 contains another "s" orbital (2 electrons) and a "p" orbital (6 electrons).
Adding up those electrons (2 + 2 + 6) equals 10.
Scrolling up, let's look at the element at the end of row 2. What is its number?
Yes, neon is element number 10! (This is the sum of the electrons in two "s" orbitals and one "p" orbital.)
Rows 1 and 2 have 10 electrons.
Row 3 has another "s" orbital (2 electrons) and another "p" orbital (6 electrons).
10 + 2 + 6 equals 18.
Scrolling up, and looking at the element at the end of row 3, we see that it is argon, element number 18.
Looking at row 4, we encounter a new type of orbital - the "d" orbital.
The first 3 rows have 18 electrons and the fourth row gives us an additional 18
("s" orbital 2, "p" orbital 6 and "d" orbital 10) which brings the total to 36.
Scrolling up and looking at the end of row 4 we see that it is krypton, element 36.
The fifth row adds an additional 18 electrons ("s" orbital 2, "p" orbital 6, "d" oribtal 10).
18 plus 36 = 54, which is the atomic number of xenon, the element at the end of row 5.
Row 6 introduces a new type of orbital - the "f" orbital.
Adding 54 electrons to 32 (electrons in the "s", "p", "d" and "f" orbitals) sums to 86.
Element 86 is radon and is at the end of row 6.
So, we can see that as the "s" and "p" obitals are filled, this produces the "Main Group" of elements (blue and red groups).
As the "d" orbitals are filled, this produces the "Transition Elements" (green group) and
as the "f" orbitals are filled, this produces the "rare earth" elements (yellow group).
Now, you can see how (and why) the periodic table has certain elements arranged in "blocks" and "groups" and you can see the reason for the "steps".
Perhaps this has increased your understanding of the periodic table, orbitals and the elctron configuration of the elements.
|




